The background: There has been a disagreement between one of our academic users and his professor regarding the IUPAC name of a structure. The student - being a ChemAxon user - turned to us to seek help in defending his answer. So let’s see the problem, the solution and the professor's answer:
The problem:
“Hello, I used MarvinSketch to help me learn IUPAC naming. Based on what I learned, I applied that knowledge on an exam, and my professor marked it wrong. When I referred him to MarvinSketch, he says that he can not give me the grade MarvinSketch "made up" the name and it is incorrect. Is there any proof that the program is correct? What can I tell him? Thank you.
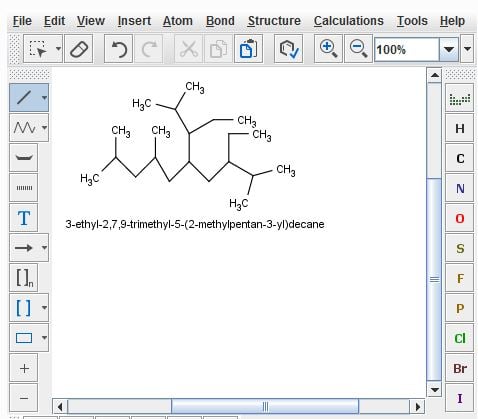 The "correct" way is 3-Ethyl-5-(1-ethyl-2-methylpropyl)-2,7,9-trimethyldecane”
The solution: "Thanks for your message. First, it should be clear that the names are identical except for the name fragment for the substituent chain: 1-ethyl-2-methylpropyl (as your professor says) or 2‐methylpentan‐3‐yl (as you and Marvin say). The difference in lexical ordering is just a consequence. It should also be clear that both names represent the same structure, but the question remains: which one is the preferred one? If we look at the
IUPAC Nomenclature of Organic Chemistry recommendations from 1979 we find this rule: 2.25 - Univalent branched radicals derived from alkanes are named by prefixing the designation of the side chains to the name of the unbranched alkyl radical possessing the longest possible chain starting from the carbon atom with the free valence, the said atom being numbered as 1. In this case, applying this rule leads to your professor's name: 1-ethyl-2-methylpropyl. Bad news for us :( Unless...
IUPAC published new nomenclature rules in 2013? Of relevance here is section
P-29.2 page 352, which presents method 1 (your professor's) and method 2, as well as section P-29.3.2.1 and P-29.3.2.2 on
page 355. There it is specified that method 1 is preferred only for "
simple substituent groups derived from acyclic hydrocarbons with a single free valence at the end of the longest chain", and method 2 is preferred in all other cases. In our case, the longest chain in the substituent has 5 carbon atoms, and its free valence is not at the end. Therefore, method 2 should be used. The
examples on page 542 confirm that interpretation, since it's the names on the left, built using method 2, that are preferred. In other words, I think your professor is right when following the 1979 IUPAC rules, and you and Marvin are right when following the 2013 rules. In this light, and since this is 2014, I think you should definitely get the highest grade! Best regards, Daniel
ChemAxon, supporting chemistry students since 1998"
The reaction of the prof: Just a couple of days after our reply to the problem, our user wrote back to us completing the story: "Thanks for your help, I convinced him to give me the mark. I would like to thank you once more for your reply."
The "correct" way is 3-Ethyl-5-(1-ethyl-2-methylpropyl)-2,7,9-trimethyldecane”
The solution: "Thanks for your message. First, it should be clear that the names are identical except for the name fragment for the substituent chain: 1-ethyl-2-methylpropyl (as your professor says) or 2‐methylpentan‐3‐yl (as you and Marvin say). The difference in lexical ordering is just a consequence. It should also be clear that both names represent the same structure, but the question remains: which one is the preferred one? If we look at the
IUPAC Nomenclature of Organic Chemistry recommendations from 1979 we find this rule: 2.25 - Univalent branched radicals derived from alkanes are named by prefixing the designation of the side chains to the name of the unbranched alkyl radical possessing the longest possible chain starting from the carbon atom with the free valence, the said atom being numbered as 1. In this case, applying this rule leads to your professor's name: 1-ethyl-2-methylpropyl. Bad news for us :( Unless...
IUPAC published new nomenclature rules in 2013? Of relevance here is section
P-29.2 page 352, which presents method 1 (your professor's) and method 2, as well as section P-29.3.2.1 and P-29.3.2.2 on
page 355. There it is specified that method 1 is preferred only for "
simple substituent groups derived from acyclic hydrocarbons with a single free valence at the end of the longest chain", and method 2 is preferred in all other cases. In our case, the longest chain in the substituent has 5 carbon atoms, and its free valence is not at the end. Therefore, method 2 should be used. The
examples on page 542 confirm that interpretation, since it's the names on the left, built using method 2, that are preferred. In other words, I think your professor is right when following the 1979 IUPAC rules, and you and Marvin are right when following the 2013 rules. In this light, and since this is 2014, I think you should definitely get the highest grade! Best regards, Daniel
ChemAxon, supporting chemistry students since 1998"
The reaction of the prof: Just a couple of days after our reply to the problem, our user wrote back to us completing the story: "Thanks for your help, I convinced him to give me the mark. I would like to thank you once more for your reply."
 The "correct" way is 3-Ethyl-5-(1-ethyl-2-methylpropyl)-2,7,9-trimethyldecane”
The solution: "Thanks for your message. First, it should be clear that the names are identical except for the name fragment for the substituent chain: 1-ethyl-2-methylpropyl (as your professor says) or 2‐methylpentan‐3‐yl (as you and Marvin say). The difference in lexical ordering is just a consequence. It should also be clear that both names represent the same structure, but the question remains: which one is the preferred one? If we look at the
IUPAC Nomenclature of Organic Chemistry recommendations from 1979 we find this rule: 2.25 - Univalent branched radicals derived from alkanes are named by prefixing the designation of the side chains to the name of the unbranched alkyl radical possessing the longest possible chain starting from the carbon atom with the free valence, the said atom being numbered as 1. In this case, applying this rule leads to your professor's name: 1-ethyl-2-methylpropyl. Bad news for us :( Unless...
IUPAC published new nomenclature rules in 2013? Of relevance here is section
P-29.2 page 352, which presents method 1 (your professor's) and method 2, as well as section P-29.3.2.1 and P-29.3.2.2 on
page 355. There it is specified that method 1 is preferred only for "
simple substituent groups derived from acyclic hydrocarbons with a single free valence at the end of the longest chain", and method 2 is preferred in all other cases. In our case, the longest chain in the substituent has 5 carbon atoms, and its free valence is not at the end. Therefore, method 2 should be used. The
examples on page 542 confirm that interpretation, since it's the names on the left, built using method 2, that are preferred. In other words, I think your professor is right when following the 1979 IUPAC rules, and you and Marvin are right when following the 2013 rules. In this light, and since this is 2014, I think you should definitely get the highest grade! Best regards, Daniel
ChemAxon, supporting chemistry students since 1998"
The reaction of the prof: Just a couple of days after our reply to the problem, our user wrote back to us completing the story: "Thanks for your help, I convinced him to give me the mark. I would like to thank you once more for your reply."
The "correct" way is 3-Ethyl-5-(1-ethyl-2-methylpropyl)-2,7,9-trimethyldecane”
The solution: "Thanks for your message. First, it should be clear that the names are identical except for the name fragment for the substituent chain: 1-ethyl-2-methylpropyl (as your professor says) or 2‐methylpentan‐3‐yl (as you and Marvin say). The difference in lexical ordering is just a consequence. It should also be clear that both names represent the same structure, but the question remains: which one is the preferred one? If we look at the
IUPAC Nomenclature of Organic Chemistry recommendations from 1979 we find this rule: 2.25 - Univalent branched radicals derived from alkanes are named by prefixing the designation of the side chains to the name of the unbranched alkyl radical possessing the longest possible chain starting from the carbon atom with the free valence, the said atom being numbered as 1. In this case, applying this rule leads to your professor's name: 1-ethyl-2-methylpropyl. Bad news for us :( Unless...
IUPAC published new nomenclature rules in 2013? Of relevance here is section
P-29.2 page 352, which presents method 1 (your professor's) and method 2, as well as section P-29.3.2.1 and P-29.3.2.2 on
page 355. There it is specified that method 1 is preferred only for "
simple substituent groups derived from acyclic hydrocarbons with a single free valence at the end of the longest chain", and method 2 is preferred in all other cases. In our case, the longest chain in the substituent has 5 carbon atoms, and its free valence is not at the end. Therefore, method 2 should be used. The
examples on page 542 confirm that interpretation, since it's the names on the left, built using method 2, that are preferred. In other words, I think your professor is right when following the 1979 IUPAC rules, and you and Marvin are right when following the 2013 rules. In this light, and since this is 2014, I think you should definitely get the highest grade! Best regards, Daniel
ChemAxon, supporting chemistry students since 1998"
The reaction of the prof: Just a couple of days after our reply to the problem, our user wrote back to us completing the story: "Thanks for your help, I convinced him to give me the mark. I would like to thank you once more for your reply."
Related content
15 12 2025
2 minutes
Chemaxon’s response on CVE-2025-55182-React2Shell
Chemaxon’s response on CVE-2025-55182-React2Shell
09 12 2025
2 minutes
Changes to Our Long-Term Support (LTS) Release Schedule
Chemaxon's long-term support (LTS) release schedule changes starting January 1st, 2026.
26 11 2025
2 minutes
Retiring Chemicalize Pro Services, Transitioning to Modern Solutions
Retiring Chemicalize Pro on December 31st, 2025, moving to more modern solutions.