Boost analytical experiments with phys-chem properties
Physicochemical properties have a fundamental impact on analytical experiment conditions, especially in case of chromatography separations. According to the nature of chemistry, dissolved compounds adapt to the environment and behave as molecular chameleons, by changing their properties in different solvents and at different pH values.
This phenomenon is reflected in the changes of tautomeric and ionization states. A simple compound with moieties that are prone to act as proton donors like acids or proton acceptors, like bases, might be present in the solution as an array of distinguished, charged microspecies. The relative abundance of these microspecies changes with the pH of the solution. Distribution of the micropecies can rapidly become a complex function of pH, driven by all the dissociation equilibrium constants. Since ionization has a characteristic role on interactions with the solvent and solvation energetics, this phenomenon translates into pH dependent changes of the retention time in case of chromatography. This well known phenomenon includes the retention time increase in case of basic compounds and decrease in acidic compounds in reverse phase HPLC chromatography [ ref1 , ref2, ref3, ref4, ref5, ref6 ] upon scanning the pH range. Variability of retention times for a single compound results in variability of the separation in case of mixtures of analytes.
Isoelectric point (pI), pKa values or pH dependent change of the lipophilicity, expressed by octanol-water partitioning coefficient (logP and logD) correlates with the retention time. As an example we show on Figure 1. the predicted logD-pH plot of ten analytes (barbital, acetylsalicylic acid, ciprofloxacine, piroxicam, indapamide, metoprolol, tramadol, cetirizine, verapamil, hydroxizine). The order of lipophilicity changes and pH ranges can be identified with low and high differences in their calculated values, which translates into differences in retention times.
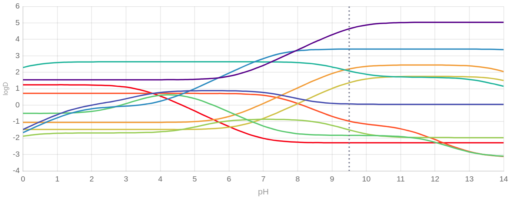
Figure 1. Calculated logD values for 10 analytes (barbital, acetylsalicylic acid, ciprofloxacine, piroxicam, indapamide, metoprolol, tramadol, cetirizine, verapamil, hydroxizine) from Ref7.
According to Paweł Wiczling et al. [Ref7, see Fig 12.3], for these 10 compounds the observed retention times followed similar tendencies and the maximal resolution could be achieved on higher pH values.
Calculation of physicochemical parameters can therefore increase the efficiency of method development and contribute to successful design of experimental conditions. Knowledge on microspecies, pI values or lipophilicity contributes to the understanding of experimental results in challenging cases.
Related content
Predicting pKa
One of the most important physicochemical properties of small molecules and macromolecules are the...
Coupling stabilizers open KV1-type potassium channels
ABSTRACT: The opening and closing of voltage-gated ion channels are regulated by voltage sensors...
Cheminfo Stories 2021 Virtual UGM | Boost analytical experiments with phys-chem properties
TRY CHEMICALIZE Log in for videos & slides
Responding to the Challenge Posed by the Generic Control of Substances
Drug monitoring organizations report that new psychoactive substances continue to emerge, posing...