Collaborating with CROs within the DMTA cycle
Important factors when collaborating with a CRO
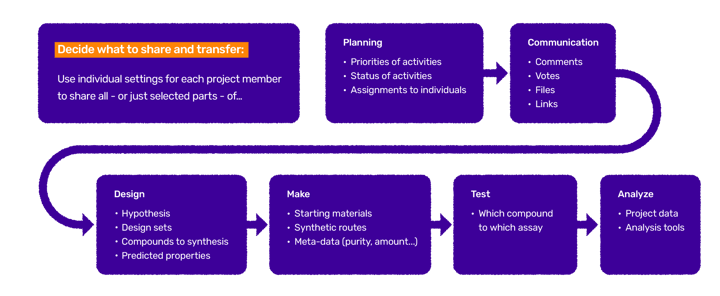
It is becoming increasingly popular for drug discovery companies to outsource parts of the DMTA process to Contract Research Organizations (CROs). This puts a higher pressure on the project leader, who needs to keep track of both in house and external activities. Having all project-related information such as data, status of ongoing activities and upcoming plans for both internal and external project members collected in one place facilitates the overview and communication.
Restricted sharing of project data
In many cases the drug discovery companies want to restrict the sharing of internal project-related information with the CRO to a need-to-know basis.
To support any kind of collaboration level within a project, Design Hub allows for individual settings of access to project information. For instance, the drug discovery company can choose to share all - or just selected - molecules for synthesis in a project. Smooth transfer of data from the CRO to the company is also important (Figure 1).
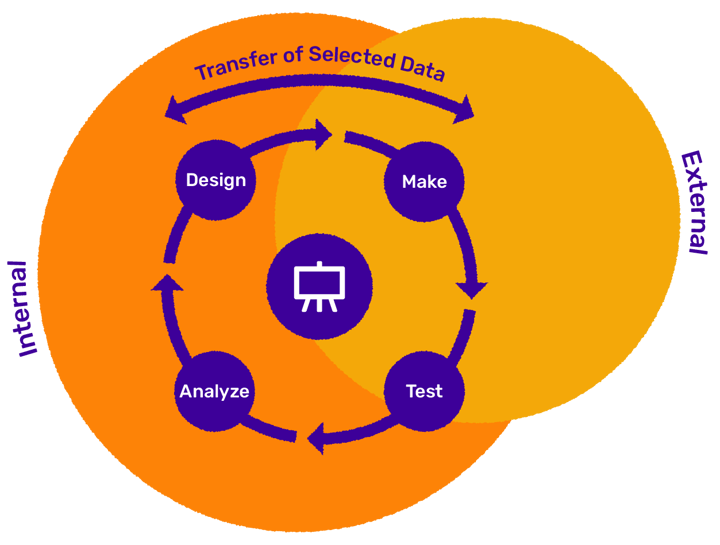
Figure 1. Depending on the level of collaboration, the CRO companies are more or less involved in the different parts of the DMTA process. Each company and project have different needs for giving the CROs access to reading and transferring data and structures.
Example of a Synthesis Tracking Overview
… from the Drug Discovery company Perspective
![]()
You can also use these fields to communicate within your own organization.
… and from the CRO Perspective
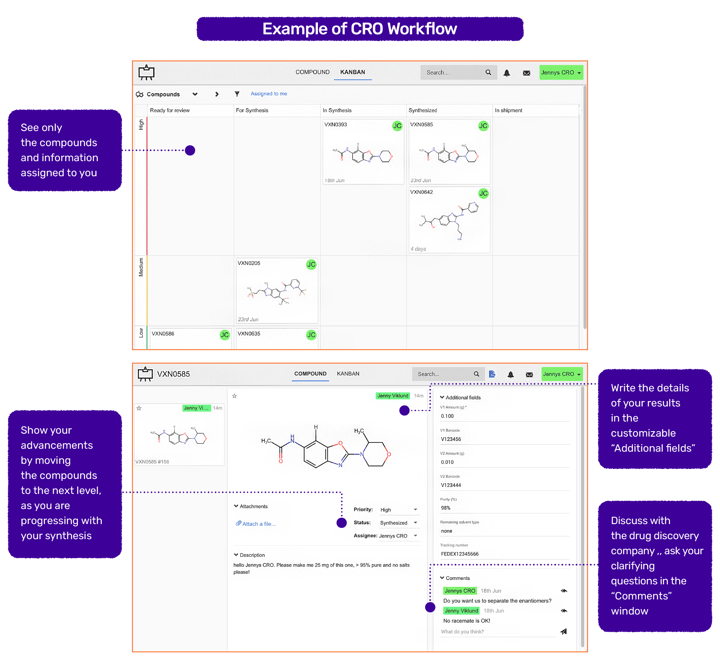 Depending on the level of collaboration, the CRO can also use this tool to track assay requests or add their own designed compounds to the KanBan board
Depending on the level of collaboration, the CRO can also use this tool to track assay requests or add their own designed compounds to the KanBan board
Related content
Certainty Discovery. Frankfurt, November 4-5, 2025
Certainty Discovery 2025 review by Wendy A. Warr - Vibes, topics and key takeaways.
The Rise of Biologics Discovery: From Small Molecules to Sequences
Biologics in drug discovery is becoming more important by the year. See where biologics discovery...
Tautomer Generation Methods – Case Study on Reliability
Tautomer data handling in a reliable manner - case study based on thorough testing to find the most...